Weight Loss and GLP-1 Side Effects


Understanding GLP-1 Medications
GLP-1 receptor agonists like Ozempic, Mounjaro, and Wegovy have revolutionized weight loss treatment by mimicking natural hormones that regulate appetite and blood sugar. These medications work by slowing stomach emptying, increasing feelings of fullness, and reducing hunger signals in the brain. While highly effective for many patients, helping them lose 10-20% of body weight, they're not free from side effects. Most side effects are related to how these drugs affect your digestive system and metabolism. Understanding potential issues can help you prepare for and manage them while working with your healthcare provider.

Nausea and Digestive Issues
Gastrointestinal problems are the most common side effects, affecting up to 40% of patients. Nausea, vomiting, diarrhea, constipation, and abdominal pain typically peak during the first few weeks of treatment or after you increase a dose. For most people, these symptoms improve as your body adjusts. Eating smaller, more frequent meals, avoiding fatty or spicy foods, and staying well-hydrated can help manage these symptoms. If digestive issues are severe or long-lasting, your doctor may recommend temporarily pausing dose increases or trying anti-nausea medications.

Taste Changes and Food Aversions
Many GLP-1 users report that foods taste different or that they no longer like foods they previously enjoyed. Sweet and fatty foods often become particularly unappetizing, which can help you eat healthier. Some patients say certain foods taste metallic, or find that their favorite beverages no longer appeal to them. While mostly temporary, some taste alterations may last as long as you're on the drugs. Some people welcome this, as it helps them naturally avoid high-calorie foods.
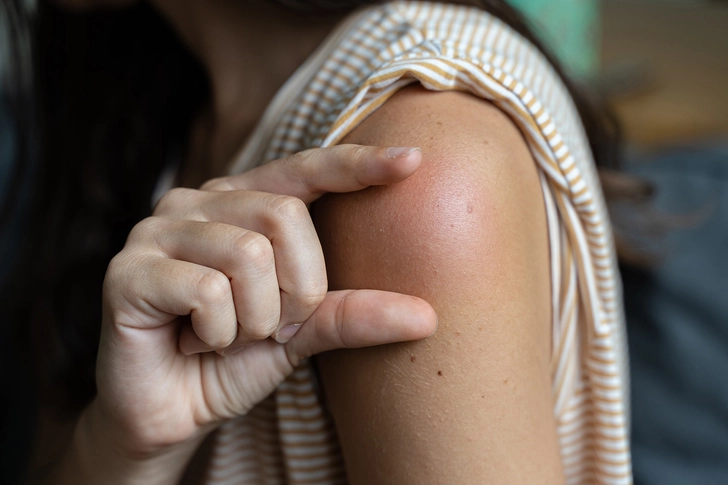
Injection Site Reactions
Since GLP-1 medications are administered via injection, some patients experience redness, itching, swelling, or bruising at injection sites. These reactions are typically mild and get better within a few days. Try rotating injection sites and avoid injecting into areas with bruises, scars, or stretch marks. Using proper injection technique and cleaning the site with alcohol before injection can also help.
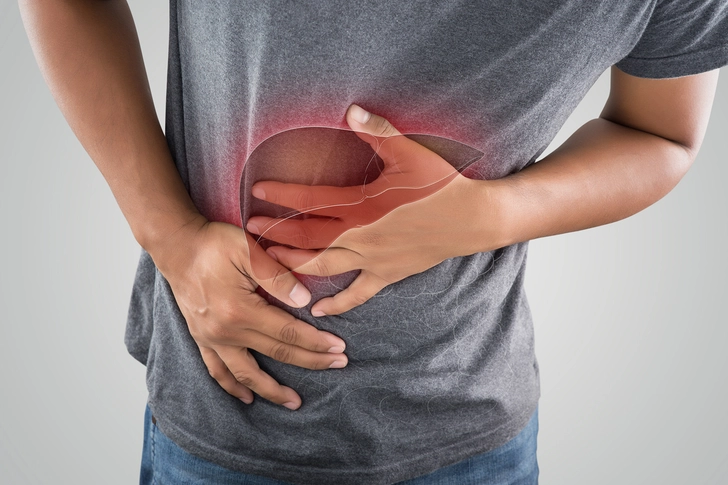
Pancreas Problems
Though rare, acute pancreatitis is a serious potential side effect of GLP-1 medications. Symptoms include severe, persistent abdominal pain that may radiate to your back, nausea, vomiting, and fever. This requires immediate medical attention. People with a history of pancreatitis or gallbladder disease may be at higher risk. Your doctor should monitor your pancreatic enzymes through blood tests during treatment. If you develop symptoms, stop taking the medication and seek emergency care.

Muscle Loss
People lose both fat and muscle when taking GLP-1 medications. Studies show that without proper exercise and nutrition, up to 25% of total weight loss may come from muscle rather than fat. This is concerning because muscle plays vital roles in metabolism, strength, and overall health. To preserve muscle while losing fat, eat enough protein, do regular resistance training at least twice weekly, and don't restrict your calories too severely. Your doctor may recommend working with a registered dietitian.

Gallbladder Complications
Rapid weight loss from any cause, including GLP-1 medications, increases the risk of gallstones and gallbladder disease. The liver secretes more cholesterol during weight loss, which can form stones in the gallbladder. Let your healthcare provider know right away if you have any of these symptoms: Your skin or the whites of your eyes turning yellowish in color (also called jaundice); Fever; Pain in your upper stomach area; Stool (poop) that is clay-colored.

Hair Thinning
Some people notice temporary hair thinning or hair loss several months after starting GLP-1 medications. This is typically due to a condition called telogen effluvium, where significant weight loss, hormonal changes, or nutritional shifts trigger hair follicles to enter a resting phase. This type of hair loss is usually reversible once weight stabilizes. Make sure you get enough protein, iron, zinc, and vitamins. Talk to your doctor if you're concerned about your hair loss.

Mood Changes
Some people taking GLP-1s notice a change in their mood, such as depression, anxiety, or unusual irritability. These effects may result from hormonal shifts, changes to your diet, or other factors. Some patients also say they can no longer tolerate alcohol or no longer want to drink. While rare, some people have reported suicidal thoughts. Let your healthcare provider know about any changes in your mental well-being. If you have an existing mental health condition, talk about the risks with your doctor before you start treatment.

Thyroid Concerns
There's a small, potential risk of thyroid cancer when taking GLP-1s. Let your doctor know right away if you have symptoms like voice changes, difficulty swallowing, shortness of breath, or a lump in the neck.

When to Seek Help
Seek medical attention right away if you have: severe, persistent abdominal pain, especially with vomiting; signs of allergic reaction like rash, itching, or swelling of face/throat; unusual or severe headaches; vision changes; persistent vomiting or inability to keep fluids down; severe diarrhea or constipation; yellowing of skin or eyes; dark urine; extreme fatigue or weakness; or suicidal thoughts.
PHOTO CREDENTIALS
Slide 1 - Moment / Getty Images
Slide 2 - Hryshchyshen Serhii / Shutterstock
Slide 3 - iStock / Getty Images
Slide 4 - Troyan / Shutterstock
Slide 5 - iStock / Getty Images
Slide 6 - Prostock-studio / Shutterstock
Slide 7 - Peakstock / Shutterstock
Slide 8 - Oporty786 / Shutterstock
Slide 9 - THICHA SATAPITANON / Shutterstock
Slide 10 - PeopleImages.com - Yuri A / Shutterstock
Slide 11 - Phynart Studio / Getty Images
SOURCES
Eli Lilly and Company: “Mounjaro (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for the treatment of adults with type 2 diabetes.”
FDA: “FDA Approves New Medication for Chronic Weight Management,” “Mounjaro (tirzepatide) Highlights of Prescribing Information,” “Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity,” “MOUNJAROTM (tirzepatide) Injection, for subcutaneous use.”
Phelps Health: “Understanding Mounjaro: A Guide for Type 2 Diabetes Patients.”
University of Colorado Health: “What is Mounjaro? And does it work better for weight loss than Ozempic and Wegovy?”
Cleveland Clinic: “How Mounjaro Is Helping People With Obesity Lose Weight,” “Gastroparesis.”
The New England Journal of Medicine: Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes.”
HCP Live: “Management of Nausea Resulting from GLP1 Agonists for T2DM.”
Harvard Medical School: “GLP-1 diabetes and weight-loss drug side effects: "Ozempic face" and more.”
Journal of Clinical Medicine: “Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus.”
Houston Methodist: “Home Remedies for Heartburn: 8 Ways to Get Rid of Acid Reflux.”
International Journal of Clinical Pharmacology: “Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database.”
Mayo Clinic: “Tirzepatide (subcutaneous route),” “Pancreatitis.”
Frontiers in Endocrinology: “Safety issues of tirzepatide (pancreatitis and gallbladder or biliary disease) in type 2 diabetes and obesity: a systematic review and meta-analysis.”
Medscape: “Tirzepatide (Rx).”
University of British Columbia: “Weight-loss drugs linked to stomach paralysis, other serious gastrointestinal conditions.”
American Diabetes Association: “Tirzepatide Slowed Progression of Chronic Kidney Disease in Patients with Type 2 Diabetes with Increased Cardiovascular Risk.”
Clinical Kidney Journal: “Tirzepatide and prevention of chronic kidney disease.”
Johns Hopkins Medicine: “Diabetes and Your Eyes: What You Need to Know,” “Gallstones.”
American Academy of Ophthalmology: “6 Surprising Facts About Diabetes and Your Eyes.”
NEJM Journal Watch: “Gastrointestinal Adverse Events in Patients Taking GLP-1 Agonists for Weight Loss.”
Clinical Pharmacology and Therapeutics: “Incretin-Based Drugs and Risk of Intestinal Obstruction Among Patients with Type 2 Diabetes.”
National Library of Medicine: “Intestinal obstruction and Ileus.”
Asthma and Allergy Foundation of America: “Anaphylaxis (Severe Allergic Reaction).”
FDA: “Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss,” “Ozempic: Highlights of prescribing information,” “Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity,” “MPM: V-9. Fruits and Fruit Products (V-51 to V-78).”
Mayo Clinic: “How does semaglutide work?” “Constipation,” “Pancreatitis,” “Gastroparesis,” “Heartburn,” “Semaglutide: Subcutaneous Route,” “Belching, gas and bloating: Tips for reducing them.”
The New England Journal of Medicine: “Once-Weekly Semaglutide in Adults with Overweight or Obesity.”
Ozempic: “Possible side effects of Ozempic.”
UCLA Health: “Semaglutide for weight loss – what you need to know.”
Diabetes, Obesity and Metabolism: “Gastrointestinal tolerability of once‐weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss.”
Molecules: “Role of Sulfur Compounds in Vegetable and Mushroom Aroma.”
Journal of Food Composition and Analysis: “Sulfur content in foods consumed in an Italian population and impact of diet quality on sulfur intake.”
St. Luke’s Health System Kansas City: “Understanding Post-Injection Inflammation.”
Novo Nordisk: “Ozempic semaglutide injection: Highlights of prescribing information.”
Houston Methodist: “Home Remedies for Heartburn: 8 Ways to Get Rid of Acid Reflux.”
Antioxidants and Redox Signaling: “Hydrogen Sulfide Signaling in the Gastrointestinal Tract.”
Cleveland Clinic: “Gastroparesis.”
University of British Columbia: “Weight-loss drugs linked to stomach paralysis, other serious gastrointestinal conditions.”
Ozempic: “Important safety information.”
American Academy of Allergy, Asthma, and Immunology: “Angioedema after semaglutide.”
Novomedlink: “Safety profile for once-weekly Ozempic (semaglutide) injection.”
Baton Rouge General: “Side Effects of Ozempic and Similar Drugs You Should Be Concerned About.”
Diatribe: “FDA Updates Ozempic Label With Warning for Intestinal Blockages.”
Frontiers in Endocrinology: “Safety of Semaglutide updated.”
Nature: “Ozempic keeps wowing: trial data show benefits for kidney disease,” “Does Ozempic boost fertility? What the science says.”
Cedars-Sinai: “Good Morning America: Is Ozempic Safe to Take During Pregnancy or While Trying to Conceive? Experts Weigh In.”
Menopause: “Weight loss response to semaglutide in postmenopausal women with and without hormone therapy use.”
National Institutes of Health: “People taking semaglutide had lower risk of suicidal thoughts.”
Asthma and Allergy Foundation of America: “Anaphylaxis (Severe Allergic Reaction).”
Harvard Medical School: “GLP-1 diabetes and weight-loss drug side effects: ‘Ozempic face’ and more.”
National Health Service (U.K.): “Feeling Sick (Nausea).”
Carolynn Francavilla Brown, MD, obesity specialist; diplomate, Board of Obesity Medicine; owner, Green Mountain Partners for Health and Colorado Weight Care, Denver.
International Archives of Otolaryngology: "The Impact of Acute Loss of Weight on Eustachian Tube Function."
Endocrine 2024, annual meeting of the Endocrine Society, Boston, June 1, 2024.
Mojca Jensterle Sever, PhD, University Medical Center, Ljubljana, Slovenia.
Megan Melo, MD, family and obesity medicine doctor, Phinney Primary Care and Wellness, Seattle.
Sofia Spieler, Boston.
Diabetes & Metabolism Journal: “Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets?”
Nutrition Reviews: “Temporal patterns in taste sensitivity.”
Cleveland Clinic: “How To Give Yourself a Subcutaneous Injection,” "Semaglutide Injection."
Great Ormond Street Hospital for Children: “Giving subcutaneous injections.”
Mount Sinai: “Subcutaneous (SQ) injections.”
National Institutes of Health: “Giving a subcutaneous injection.”
Vancouver Coastal Health: “How to give yourself a Subcutaneous Injection: A Guide for Patients.”
Johns Hopkins Arthritis Center: “How to Give a Subcutaneous Injection.”
LibreTexts Medicine: “18.5: Administering Subcutaneous Medications.”
FDA: “Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss,” "FDA alerts health care providers, compounders and patients of dosing errors associated with compounded injectable semaglutide products,” "Human Drug Compounding."
Zepbound: “Take Zepbound at home, with or without food, once a week.”
CDC: “Preventing Unsafe Injection Practices,” "4 Ways to Take Insulin."
Mayo Clinic Health System: “Get to the point: Proper disposal of sharps,” "Semaglutide (Subcutaneous route)," "How to inject semaglutide for weight loss," "What Happens if You Take Too Much Semaglutide?"
UW Health: “Subcutaneous Injection.”
Saint Luke’s: “Insulin: How to Use and Where to Inject.”
University of Maryland Baltimore County: “Innies, outies and omphalophobia: 7 navel-gazing questions about belly buttons answered.”
Tubbs, R., editor. Nerves and Nerve Injuries: Vol 2, Elsevier, 2015: “Nerve Compression/Entrapment Sites of the Lower Limb.”
American Diabetes Association: "Insulin Storage and Syringe Safety."
Toxics: "The Other Face of Insulin—Overdose and Its Effects."
Eli Lilly: "Instructions for Use: Zepbound (tirzepatide) injection for subcutaneous use."
Farhad Mehrtash, MPH, co-author, GLP-1 weight loss medication guidelines, Harvard T.H. Chan School of Public Health.
JoAnn E. Manson, MD, professor of medicine, Harvard Medical School.
Samuel Klein, MD, professor of medicine and nutritional science, Washington University, St. Louis.
Jody Dushay, MD, assistant professor of medicine, Harvard Medical School.
JAMA Internal Medicine: "Integrating Diet and Physical Activity When Prescribing GLP-1s – Lifestyle Factors Remain Crucial," "I Am Taking a GLP-1 Weight-Loss Medication – What Should I Know?"
CDC: "Adult Activity: An Overview."
The National Weight Control Registry: "NWCR Facts."
Brian Abittan, MD, director of Skin and Hair Rejuvenation and director of Hair Transplantation, Kimberly and Eric J. Waldman Department of Dermatology, Mount Sinai, New York City.
Mayo Clinic: “Hair loss.”
American Academy of Dermatology: “Hair Loss Types: Alopecia Areata Signs and Symptoms,” “Hair Loss: Who Gets and Causes,” “Hair Loss: Signs and Symptoms,” “Hair Loss: Tips for Managing.”
NYU Langone Health: “Types of Hair Loss,” “Medication for Hair Loss.”
Cleveland Clinic: “Folliculitis Decalvans,” “Hair Loss,” “Hair Loss Treatments, “Scalp Micropigmentation,” “Does Creatine Cause Hair Loss?” “When to Worry about Hair Loss.”
Michael Pan, MD, dermatologist who specializes in hair loss, Cedars Sinai, Los Angeles.
Harvard Nutrition Source: “Biotin - Vitamin B7.”
Medscape: “Does Ozempic Cause Hair Loss?”
Medical News Today: “Ozempic, Wegovy Under Investigation for Side Effects of Suicide Ideation, Hair Loss.”
American Society for Dermatologic Surgery.
American Academy of Family Physicians.
Wegovy (Novo Nordisk) US Medication Guide, August 2025.
Wegovy (Novo Nordisk) US Prescribing Information, August 2025.