Weight Loss and GLP-1 Side Effects


Understanding GLP-1 Medications
GLP-1 receptor agonists (Ozempic, Wegovy, Mounjaro, and Zepbound) have transformed weight loss by mimicking glucagon-like peptide-1, a hormone naturally released after eating. These medications work by doing the following: they slow stomach emptying, which creates longer-lasting feelings of fullness; increase insulin secretion while decreasing glucagon (improving blood sugar control); and act directly on appetite centers in the brain to reduce hunger and food cravings. While highly effective—helping people lose 15% to 20% of their weight—they change your body's metabolism and digestion while you're on them. This explains why side effects are common, with most people having at least mild symptoms. Most side effects happen in the first few weeks of treatment or after you increase your dose. Keep in mind that these medications are meant to be used long-term use, and you're likely to regain weight if you stop taking them.

Nausea and Digestive Issues
Gastrointestinal symptoms are the most frequently reported side effects, affecting up to 44% of patients taking GLP-1 medications. Nausea is most common, followed by vomiting, diarrhea, constipation, and abdominal pain or discomfort. This is because GLP-1s slow down the movement of food through your stomach and gut. Most people find these symptoms are worst during the first 4-8 weeks of treatment or after you increase the dose. It can help if you eat smaller, more frequent meals rather than three large ones; avoid fatty, spicy, or high fiber foods during initial treatment; drink plenty of water; try ginger tea or peppermint for natural nausea relief; and take the medication at bedtime if daytime symptoms are bothering you. Some people may find relief from anti-nausea medications when they first start taking GLP-1s. If your symptoms are severe or last longer than 8 weeks, your doctor might temporarily reduce your dose before increasing it again more gradually.

Taste Changes and Food Aversions
Many GLP-1 users report that foods taste different or that they no longer like foods they previously enjoyed. They may no longer enjoy their favorite foods, or be extra sensitive to sweetness, metallic or bitter taste sensations, and they make strongly dislike certain foods and drinks —particularly sweets, fatty foods, and alcohol. Some people describe meat as suddenly tasting "gamey" or say that coffee tastes unpleasantly bitter. Many people view these changes in their tastes as beneficial "side effects" that help them eat less high-calorie food. For some people, these changes may last as long as they are taking GLP-1s. Try using herbs and spices to enhance flavor, trying new foods you previously disliked, focus on protein sources you can still tolerate, and stay flexible with your meal planning because your tastes may change from day to day.
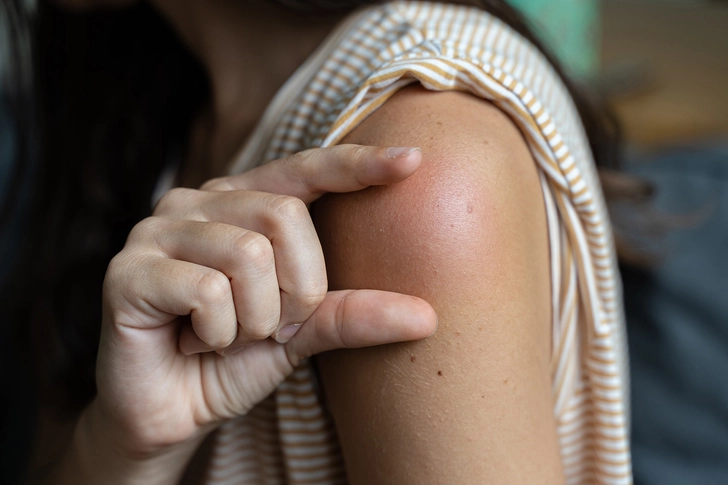
Injection Site Reactions
GLP-1 medications are injected under the skin, and some people may have a reaction at the injection site, such as redness, itching, swelling, bruising, or occasionally small bumps under the skin. Most reactions are mild and get better within 3-5 days without treatment. Try rotating injection sites and avoid injecting into areas with bruises, scars, or stretch marks. Make sure the medication is at room temperature before injecting (cold medication increases discomfort); clean the site thoroughly with alcohol and allow it to dry completely; inject at a 90-degree angle with quick, confident motion; and avoid injecting into areas with scars, stretch marks, moles, or visible blood vessels. Using ice briefly before injection can reduce pain, while applying a cold compress afterward can minimize swelling. If you develop a rash, severe pain, warmth, or drainage at an injection site, tell your healthcare provider promptly as these may indicate infection.
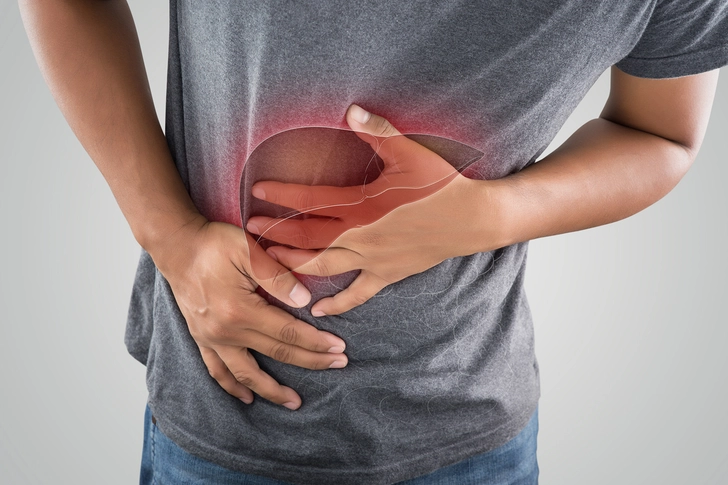
Pancreas Problems
While rare, acute pancreatitis (an inflamed pancreas) represents one of the most serious potential complications of GLP-1 medication use, affecting approximately 0.1-0.2% of people. Seek medical attention immediately if you have severe, persistent abdominal pain that often radiates to the back, accompanied by nausea, vomiting, and sometimes fever. You're at higher risk if you have a history of pancreatitis, gallstones, heavy alcohol use, or very high triglyceride levels. Before starting treatment, your doctor should evaluate your baseline pancreatic enzyme levels through blood tests and continue monitoring periodically. You'll have to stop taking the GLP-1 if you have pancreatitis.

Muscle Loss
People lose both fat and muscle when on GLP-1s. Research suggests that up to 20-30% of total weight loss may come from muscle rather than fat—a problem since muscle is so important for your metabolism, long-term weight maintenance, and everyday life. You lose muscle mainly because GLP-1 medications reduce overall calorie intake, often dramatically, so you may not be getting enough protein. To minimize muscle loss while on these medications, get enough protein (approximately 100-150 grams for most adults); do resistance training 2-3 times weekly, focusing on major muscle groups; make sure you're getting enough nutrients, especially calcium and vitamin D for bone health; and consider working with a registered dietitian to develop a personalized nutrition plan. Some healthcare providers also recommend body composition analysis before and during treatment to monitor your muscle mass.

Gallbladder Complications
Rapid weight loss from GLP-1 medications increases the risk of gallbladder disease. The main problem is gallstones. These stones can cause intense pain, particularly after fatty meals. Call your health care provider right away if you have any of the following symptoms: Your skin or the whites of your eyes turning yellowish in color (also called jaundice); Fever; Pain in your upper stomach area; Stool (poop) that is clay-colored.

Hair Thinning
Hair thinning or increased shedding affects approximately 5-10% of patients on GLP-1 medications, typically beginning 3-6 months after starting treatment. You're more likely to see hair thinning rather than patchy baldness. This is a condition called telogen effluvium, where significant physiological stress—in this case, rapid weight loss and potential nutritional changes—pushes an unusually high percentage of hair follicles into their resting phase simultaneously. The good news is that this type of hair loss is almost always temporary, with regrowth typically beginning once weight stabilizes and you're getting better nutrition. To minimize hair thinning, make sure you get enough protein and iron, zinc, biotin, and vitamins A and D, which all support hair health; consider a multivitamin formulated for hair support; and avoid very low-calorie diets (under 1,000 calories daily) unless medically supervised. Gentle hair care practices also help: minimize heat styling, chemical treatments, and tight hairstyles; and handle wet hair carefully to avoid breakage. Most people see their hair start to grow again within 3-6 months after their weight stabilizes.

Mood Changes
Some people taking GLP-1s notice a change in their mood, such as depression, anxiety, or unusual irritability. These effects may result from hormonal shifts, changes to your diet, or other factors. Some people have a difficult time adjusting to the changes in their relationship with food, especially if they have a history of eating disorders. Some patients also say they can no longer tolerate alcohol or no longer want to drink. While rare, some people have reported suicidal thoughts. Let your healthcare provider know about any changes in your mental well-being. If you have an existing mental health condition, talk about the risks with your doctor before you start treatment.

Thyroid Concerns
GLP-1 medications carry a warning regarding thyroid C-cell tumors based on findings in animal studies, where these medications increased the incidence of thyroid cancer. While this effect has not been confirmed in humans, the FDA requires this warning out of an abundance of caution. Talk to your doctor if you have a personal or family history of thyroid cancer. During treatment, watch for symptoms that could indicate thyroid issues: difficulty swallowing, hoarseness or voice changes, shortness of breath, or a noticeable lump in the neck.

When to Seek Help
Contact your healthcare provider immediately for: severe, persistent abdominal pain, especially if it radiates to your back (possible pancreatitis); persistent vomiting or inability to keep fluids down for more than 24 hours (risk of dehydration); severe headache with vision changes (possible secondary effect on blood pressure); signs of allergic reaction like rash, itching, swelling of face/lips/tongue, or difficulty breathing (rare but serious); sudden, intense pain in the upper right abdomen, particularly after eating (possible gallbladder issue); unusual fatigue, weakness, or dizziness (potential hypoglycemia, especially if taking diabetes medications); yellowing of skin or eyes or dark urine (possible liver effects); persistent or severe depression, anxiety, or thoughts of self-harm (requires immediate evaluation); or any symptom that really bothers you. Don't stop taking your medication without talking to your doctor.
PHOTO CREDENTIALS
Slide 1 - Moment / Getty Images
Slide 2 - Hryshchyshen Serhii / Shutterstock
Slide 3 - iStock / Getty Images
Slide 4 - Troyan / Shutterstock
Slide 5 - iStock / Getty Images
Slide 6 - Prostock-studio / Shutterstock
Slide 7 - Peakstock / Shutterstock
Slide 8 - Oporty786 / Shutterstock
Slide 9 - THICHA SATAPITANON / Shutterstock
Slide 10 - PeopleImages.com - Yuri A / Shutterstock
Slide 11 - Phynart Studio / Getty Images
SOURCES
Eli Lilly and Company: “Mounjaro (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for the treatment of adults with type 2 diabetes.”
FDA: “FDA Approves New Medication for Chronic Weight Management,” “Mounjaro (tirzepatide) Highlights of Prescribing Information,” “Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity,” “MOUNJAROTM (tirzepatide) Injection, for subcutaneous use.”
Phelps Health: “Understanding Mounjaro: A Guide for Type 2 Diabetes Patients.”
University of Colorado Health: “What is Mounjaro? And does it work better for weight loss than Ozempic and Wegovy?”
Cleveland Clinic: “How Mounjaro Is Helping People With Obesity Lose Weight,” “Gastroparesis.”
The New England Journal of Medicine: Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes.”
HCP Live: “Management of Nausea Resulting from GLP1 Agonists for T2DM.”
Harvard Medical School: “GLP-1 diabetes and weight-loss drug side effects: "Ozempic face" and more.”
Journal of Clinical Medicine: “Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus.”
Houston Methodist: “Home Remedies for Heartburn: 8 Ways to Get Rid of Acid Reflux.”
International Journal of Clinical Pharmacology: “Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database.”
Mayo Clinic: “Tirzepatide (subcutaneous route),” “Pancreatitis.”
Frontiers in Endocrinology: “Safety issues of tirzepatide (pancreatitis and gallbladder or biliary disease) in type 2 diabetes and obesity: a systematic review and meta-analysis.”
Medscape: “Tirzepatide (Rx).”
University of British Columbia: “Weight-loss drugs linked to stomach paralysis, other serious gastrointestinal conditions.”
American Diabetes Association: “Tirzepatide Slowed Progression of Chronic Kidney Disease in Patients with Type 2 Diabetes with Increased Cardiovascular Risk.”
Clinical Kidney Journal: “Tirzepatide and prevention of chronic kidney disease.”
Johns Hopkins Medicine: “Diabetes and Your Eyes: What You Need to Know,” “Gallstones.”
American Academy of Ophthalmology: “6 Surprising Facts About Diabetes and Your Eyes.”
NEJM Journal Watch: “Gastrointestinal Adverse Events in Patients Taking GLP-1 Agonists for Weight Loss.”
Clinical Pharmacology and Therapeutics: “Incretin-Based Drugs and Risk of Intestinal Obstruction Among Patients with Type 2 Diabetes.”
National Library of Medicine: “Intestinal obstruction and Ileus.”
Asthma and Allergy Foundation of America: “Anaphylaxis (Severe Allergic Reaction).”
FDA: “Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss,” “Ozempic: Highlights of prescribing information,” “Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity,” “MPM: V-9. Fruits and Fruit Products (V-51 to V-78).”
Mayo Clinic: “How does semaglutide work?” “Constipation,” “Pancreatitis,” “Gastroparesis,” “Heartburn,” “Semaglutide: Subcutaneous Route,” “Belching, gas and bloating: Tips for reducing them.”
The New England Journal of Medicine: “Once-Weekly Semaglutide in Adults with Overweight or Obesity.”
Ozempic: “Possible side effects of Ozempic.”
UCLA Health: “Semaglutide for weight loss – what you need to know.”
Diabetes, Obesity and Metabolism: “Gastrointestinal tolerability of once‐weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss.”
Molecules: “Role of Sulfur Compounds in Vegetable and Mushroom Aroma.”
Journal of Food Composition and Analysis: “Sulfur content in foods consumed in an Italian population and impact of diet quality on sulfur intake.”
St. Luke’s Health System Kansas City: “Understanding Post-Injection Inflammation.”
Novo Nordisk: “Ozempic semaglutide injection: Highlights of prescribing information.”
Houston Methodist: “Home Remedies for Heartburn: 8 Ways to Get Rid of Acid Reflux.”
Antioxidants and Redox Signaling: “Hydrogen Sulfide Signaling in the Gastrointestinal Tract.”
Cleveland Clinic: “Gastroparesis.”
University of British Columbia: “Weight-loss drugs linked to stomach paralysis, other serious gastrointestinal conditions.”
Ozempic: “Important safety information.”
American Academy of Allergy, Asthma, and Immunology: “Angioedema after semaglutide.”
Novomedlink: “Safety profile for once-weekly Ozempic (semaglutide) injection.”
Baton Rouge General: “Side Effects of Ozempic and Similar Drugs You Should Be Concerned About.”
Diatribe: “FDA Updates Ozempic Label With Warning for Intestinal Blockages.”
Frontiers in Endocrinology: “Safety of Semaglutide updated.”
Nature: “Ozempic keeps wowing: trial data show benefits for kidney disease,” “Does Ozempic boost fertility? What the science says.”
Cedars-Sinai: “Good Morning America: Is Ozempic Safe to Take During Pregnancy or While Trying to Conceive? Experts Weigh In.”
Menopause: “Weight loss response to semaglutide in postmenopausal women with and without hormone therapy use.”
National Institutes of Health: “People taking semaglutide had lower risk of suicidal thoughts.”
Asthma and Allergy Foundation of America: “Anaphylaxis (Severe Allergic Reaction).”
Harvard Medical School: “GLP-1 diabetes and weight-loss drug side effects: ‘Ozempic face’ and more.”
National Health Service (U.K.): “Feeling Sick (Nausea).”
Carolynn Francavilla Brown, MD, obesity specialist; diplomate, Board of Obesity Medicine; owner, Green Mountain Partners for Health and Colorado Weight Care, Denver.
International Archives of Otolaryngology: "The Impact of Acute Loss of Weight on Eustachian Tube Function."
Mir Ali, MD, bariatric surgeon, medical director, MemorialCare Surgical Weight Loss Center at Orange Coast Medical Center, Fountain Valley, California.
Wesley McWhorter, DrPH, MS, RDN, LD, CSCS, spokesperson, Academy of Nutrition and Dietetics, Houston.
Paunel Vukasinov, MD, internist and obesity medicine specialist, Medical Offices of Manhattan, New York City.
Harvard Health Publishing: “How does Ozempic work? Understanding GLP-1s for diabetes, weight loss, and beyond,” “GLP-1 diabetes and weight-loss drug side effects: "Ozempic face" and more.”
Nutrients: “What Is Food Noise? A Conceptual Model of Food Cue Reactivity.”
Obesity: “GLP-1 therapy increases visceral adipose tissue metabolic activity: Lessons from a randomized controlled trial in obstructive sleep apnea.”
Diabetes Care: “Efficacy of GLP-1 Receptor Agonists on Weight Loss, BMI, and Waist Circumference for Patients With Obesity or Overweight: A Systematic Review, Meta-analysis, and Meta-regression of 47 Randomized Controlled Trials.”
Mayo Clinic: “GLP-1 Agonists,” “Why Ozempic might not be working in your weight loss journey,” “Getting past a weight-loss plateau.””
Columbia Surgery Center for Metabolic and Weight Loss Surgery: “The Ozempic Effect: Everything You Need to Know About Medical Weight Loss.”
New England Journal of Medicine: “Once-Weekly Semaglutide in Adults with Overweight or Obesity.”
Cell Metabolism: “Unexpected effects of semaglutide on skeletal muscle mass and force-generating capacity in mice.”
MD Anderson Cancer Center: “4 ways to beat a weight loss plateau.”
Brown University Health: “How To Get Through Weight Loss Plateaus and Regain.”
Cleveland Clinic: “Ozempic for Weight Loss: Who Should Try It and Will it Work?”
Endocrine 2024, annual meeting of the Endocrine Society, Boston, June 1, 2024.
Mojca Jensterle Sever, PhD, University Medical Center, Ljubljana, Slovenia.
Megan Melo, MD, family and obesity medicine doctor, Phinney Primary Care and Wellness, Seattle.
Sofia Spieler, Boston.
Diabetes & Metabolism Journal: “Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets?”
Nutrition Reviews: “Temporal patterns in taste sensitivity.”
Cleveland Clinic: “How To Give Yourself a Subcutaneous Injection,” "Semaglutide Injection."
Great Ormond Street Hospital for Children: “Giving subcutaneous injections.”
Mount Sinai: “Subcutaneous (SQ) injections.”
National Institutes of Health: “Giving a subcutaneous injection.”
Vancouver Coastal Health: “How to give yourself a Subcutaneous Injection: A Guide for Patients.”
Johns Hopkins Arthritis Center: “How to Give a Subcutaneous Injection.”
LibreTexts Medicine: “18.5: Administering Subcutaneous Medications.”
FDA: “Medications Containing Semaglutide Marketed for Type 2 Diabetes or Weight Loss,” "FDA alerts health care providers, compounders and patients of dosing errors associated with compounded injectable semaglutide products,” "Human Drug Compounding."
Zepbound: “Take Zepbound at home, with or without food, once a week.”
CDC: “Preventing Unsafe Injection Practices,” "4 Ways to Take Insulin."
Mayo Clinic Health System: “Get to the point: Proper disposal of sharps,” "Semaglutide (Subcutaneous route)," "How to inject semaglutide for weight loss," "What Happens if You Take Too Much Semaglutide?"
UW Health: “Subcutaneous Injection.”
Saint Luke’s: “Insulin: How to Use and Where to Inject.”
University of Maryland Baltimore County: “Innies, outies and omphalophobia: 7 navel-gazing questions about belly buttons answered.”
Tubbs, R., editor. Nerves and Nerve Injuries: Vol 2, Elsevier, 2015: “Nerve Compression/Entrapment Sites of the Lower Limb.”
American Diabetes Association: "Insulin Storage and Syringe Safety."
Toxics: "The Other Face of Insulin—Overdose and Its Effects."
Eli Lilly: "Instructions for Use: Zepbound (tirzepatide) injection for subcutaneous use."
National Pancreas Foundation.
American Gastroenterological Association: ''Contrary to Popular Belief, Not All Cases of Chronic Pancreatitis are Alcohol- Induced.''
National Institute of Diabetes and Digestive and Kidney Diseases: "Pancreatitis."
Mayo Clinic: "Pancreatitis."
National Health Service (UK): "Acute Pancreatitis," "Chronic Pancreatitis."
Merck Manuals Professional Version: "Overview of Pancreatitis."
Johns Hopkins Medicine: "Pancreatitis," "Pancreatic Cancer and African Americans."
Cleveland Clinic: "Pancreatitis," "Steatorrhea."
World Journal of Gastroenterology: "Alcohol consumption on pancreatic diseases."
Journal of Cancer Therapy: "Smoking and Pancreatic Disease."
Current Opinion in Gastroenterology: "Obesity and pancreatitis."
Penn Medicine: "Pancreatitis."
NHS Inform: "Acute Pancreatitis."
American Family Physician: "Acute Pancreatitis: Diagnosis, Prognosis, and Treatment."
American Addiction Centers: "Pancreatitis & Alcohol: Alcohol's Effect on the Pancreas."
JAMA: "Chronic pancreatitis," "Acute Pancreatitis: A Review."
OpenStax: "The Endocrine Pancreas."
UpToDate: "Etiology of acute pancreatitis," "Pathogenesis acute pancreatitis," "Etiology and pathogenesis of chronic pancreatitis in adults,""Patient education: Acute pancreatitis (Beyond the Basics)," "Patient education: Chronic pancreatitis (Beyond the Basics)."
American Journal of Roentgenology: "Spectrum of Causes of Pancreatic Calcifications."
Farhad Mehrtash, MPH, co-author, GLP-1 weight loss medication guidelines, Harvard T.H. Chan School of Public Health.
JoAnn E. Manson, MD, professor of medicine, Harvard Medical School.
Samuel Klein, MD, professor of medicine and nutritional science, Washington University, St. Louis.
Jody Dushay, MD, assistant professor of medicine, Harvard Medical School.
JAMA Internal Medicine: "Integrating Diet and Physical Activity When Prescribing GLP-1s – Lifestyle Factors Remain Crucial," "I Am Taking a GLP-1 Weight-Loss Medication – What Should I Know?"
CDC: "Adult Activity: An Overview."
The National Weight Control Registry: "NWCR Facts."
Brian Abittan, MD, director of Skin and Hair Rejuvenation and director of Hair Transplantation, Kimberly and Eric J. Waldman Department of Dermatology, Mount Sinai, New York City.
Mayo Clinic: “Hair loss.”
American Academy of Dermatology: “Hair Loss Types: Alopecia Areata Signs and Symptoms,” “Hair Loss: Who Gets and Causes,” “Hair Loss: Signs and Symptoms,” “Hair Loss: Tips for Managing.”
NYU Langone Health: “Types of Hair Loss,” “Medication for Hair Loss.”
Cleveland Clinic: “Folliculitis Decalvans,” “Hair Loss,” “Hair Loss Treatments, “Scalp Micropigmentation,” “Does Creatine Cause Hair Loss?” “When to Worry about Hair Loss.”
Michael Pan, MD, dermatologist who specializes in hair loss, Cedars Sinai, Los Angeles.
Harvard Nutrition Source: “Biotin - Vitamin B7.”
Medscape: “Does Ozempic Cause Hair Loss?”
Medical News Today: “Ozempic, Wegovy Under Investigation for Side Effects of Suicide Ideation, Hair Loss.”
American Society for Dermatologic Surgery.
American Academy of Family Physicians.
Wegovy (Novo Nordisk) US Medication Guide, August 2025.
Wegovy (Novo Nordisk) US Prescribing Information, August 2025.
